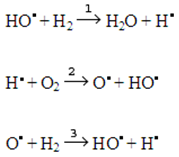|
01.01.15
Конкурс РФФИ 2015 года
Российский фонд фундаментальных исследований (РФФИ) проводит конкурс проектов участия российских ученых в научных мероприятиях, проводимых за рубежом 2012 года. Заявки принимаются до 01.11.2015 Внимание Вышла в продажу вторая редакция книги "Twenty-First Century. General Chemistry" |
Home / Доклады и выступления / Report ReportDemystifying chemical bonding, chemical kinetics and catalysis Yuriy Gankin PhD, Prof. Victor Gankin Dr. Sci.. Institute of Theoretical Chemistry, Inc., Needham, MA, United States; GGA Software Services, LLC, Cambridge, MA, United States According to J.N. Spencer (JCE No. 3 p.182 1991) base of quantum-chemical interpretations are 'unteachable'. We have developed the phenomenological (data-driven) "teachable" approach for explaining the main phenomena of chemical bonding and chemical reactions. Experimental facts that have been collected over the last fifty years lead to change in paradigms of chemical kinetics and catalysis. Meanwhile, chemical education is still largely based upon a combination of obsolete and mechanistic (non-chemical) description and involves an incomprehensible transition state entity. Our approach logically explains how reactions proceed with the breaking of the chemical bond at normal temperatures, while much higher energies are required to break such bonds thermally. It also helps to understand why radicals and ions are chemically active and how chemical interactions are facilitated by the catalysts. A comprehensible explanation of chemical bonding, kinetics and catalysis phenomena suitable for introductory chemistry program is presented [details provided at http://www.itchem.com]. In our previous papers at AC Society meetings and their generalizations in books published in 1990 - 2007, the electronic versions of which can be found at our web-site itchem.com, we described the results of our works in detail. In the course of these works, we managed to understand how chemical bond forms, how chemical reaction proceeds as well as understood catalysis mechanism. At present (2010), the final result of these works is the fact that we succeeded in understanding of the physical sense of main rules and principles discovered in the course of investigation of the above-mentioned main chemical phenomena (Lewis Rule, Resonance Rules, Valence Rules, Semenov - Polanyi, Rules, Periodic Law etc.) as well as in suggesting of an explanation of this physical sense intelligible for schoolchildren. Physical sense of the Lewis Rules. Lewis Rules stating that atoms strive to finish building their shells up to the noble gas shells have an anthropophysical sense. Experimental studies preceding to our investigations proved that electrons surrounding atomic nuclei are located in layers; number of electrons in each layer was evaluated. Analysis of these data within the Periodic Law showed that when moving along period, the nucleus charge increases by one unit and the number of electrons in the outermost electronic shell increases by one unit. This regularity had boundaries depending on the period number. For the first period, this regularity was observed for two elements only ( ); for the second and third, for 8 elements; 18 elements for the 4th and 5th periods. In the course of investigation of the chemical bond nature, we proved that during formation of a covalent chemical bond, outer electron enters the outermost shell of the other atom. That is, chemical bond formation between atoms is possible only if the outermost shells of these atoms are incomplete, i.e. the number of electrons in them is lower than maximum. Inert gases have a maximum possible number of electrons in the outermost shell. Chemical bonds formation of one atom with others stops when the number of electrons in the outermost shell of this atom is equal to the number of electrons of the nearest noble gas. Physical sense of the valence rule. Conventional quantum chemical explanations stated that elements valence is determined by the number of electrons with opposite spins in the upper electronic layer. Physical sense of the electron spin was unknown. After elucidation of the physical sense of the Lewis Rules, physical sense of the valence rules became clear. Valence is on the one hand determined by the number of electrons in the outermost shell and on the other hand, by the number of electrons that the given atom can connect to its outermost shell with energy gain. Physical sense of the Resonance Rules. Resonance Rules were used for explanation of structure of compounds, which may be described within the Lewis Rules by several structural formulas. According to the Resonance Rules, real structure of the given substance is a superposition of all structures possible within the Lewis Rules. The word "superposition" didn't have a physical sense. Within the Resonance Rules, isomerization between structures was excluded. Based on generalization of the published data on electronic isomerization, we showed that all phenomena illustrated by the Resonance Rules are explained by electronic isomerization.
Physical sense of the Semenov - Polanyi Rules. Semenov - Polanyi Rule consists in the assertion that the greater heat effect of a chemical reaction, the higher its velocity in reactions of the same type. Within the Transition State Theory, which preceded our Theory of Elementary Interactions, the physical sense of this rule was incomprehensible. Within the Theory of Elementary Interactions, it was shown that chemical reactions proceed along a chain mechanism. Atoms with incomplete outermost shells are active particles of this mechanism. Let us consider hydrogen and oxygen interaction as an example. If we place oxygen and hydrogen into a closed test-tube under room temperature, reaction between these gases will not start. However, if this mixture is subject to electric current or excited by other means, an explosion will take place. This reaction proceeds along a chain mechanism through the stages of chain initiation, propagation, and interruption. Chain initiation is a process of initial radical formation under the influence of external disturbance or presence of the third particles (for example, dusts or metals, M): Н2 + О2 → 2НО• Н2 + M → 2Н• + Me О2 + О2 → О• + О3 where М is metal. Chain propagation may be simple (when radical and molecule interaction produces one radical again) or branched (when such reaction causes production of more than one radical).
At stages 2 and 3, due to the interaction of radical and molecule, two new radicals are produced, and the process of chain branching takes place. Due to heat release, gas mixture heats up rapidly. This causes exponential reaction acceleration, which, in its turn, causes greater heat release and corresponding increase of reaction mixture temperature. Sharp and fast (momentary) temperature increase results in a similar fast and sharp pressure increase in the system, which causes an explosion. According to a general scheme of chemical interactions, energy released at previous reaction stages may be expended at chemical bonds breaking. In this case, in addition to the previously considered way of reaction, another alternative, for example, for deuterium and molecular hydrogen interaction may be assumed: Here, symbol * stands for a thermally activated molecule. Stage 4, at which breaking of a weak chemical bond in [DHH] complex takes place, proceeds with absorption of the energy released during formation of new chemical bonds at previous stages. The influence of pressure on reaction rate shows the existence of such reaction way. When pressure increases, energy dissipation rate grows due to the increase of molecule number in a reaction volume unit, i.e. concentration growth. This, in its turn, causes reduction of active particles share in the mixture. Reactions, in which energy released at exothermic stages is further expended at subsequent endothermic steps, may be called chemically activated reactions. Most of radical reactions with unsaturated molecules (with double and triple bonds) belong to such interactions. Main feature of these reactions is their independence of temperature, as the activation energy of these reactions is usually equal to zero. Number of electronic isomers with weak initial chemical bond is determined by the difference between initial and formed during reaction bond energy. The higher energy of a new bond formed during reaction, the higher concentration of this isomer in an equilibrium mixture according to thermodynamics.
Physical sense of a catalyst role in chemical reactions According to the chemical reaction mechanism, reaction rate is determined by concentration of intermediate compound and active particles. Catalysts may be conventionally divided into two groups. The first group includes substances, which produce considerably more active particles carrying out a chain process under reaction conditions. Alkaline and acid hydrolysis of esters is a demonstrative example. Catalysts increasing concentration of an intermediate compound refer to the second group. These catalysts form a complex with both saturated molecules; electronic isomerization proceeds via an intermediate formation of chemical bonds with catalyst. Moreover, the role of chemical activation increases with energy gain during reaction; this allows understanding the physical sense of the Semenov - Polanyi Rules. Physical sense of the Periodic Law According to Mendeleev's statement, elements properties periodically change when atomic weight increases. After Moseley's works, this law was formulated as follows: elements properties periodically change when the nuclear charge increases. Periodic Law was a great discovery systematizing both elements and elementary and complex substances composed of these elements. Moreover, it allowed Mendeleev to predict physical properties of so far undiscovered elements. After Rutherford's discovery of atom structure, the Periodic Law became a paradoxical law, equal to the Law of Equality of Inertial and Gravitational Mass by Newton after its paradoxicality level. The physical sense is cause and effect relationship between phenomena. Paradoxicality of these laws consisted in the fact that it was impossible even to propose as an absurd hypothesis any cause and effect relationship for explanation of periodical dependence of physical and chemical properties of elements and substances composed of them on the charge of their nuclei surrounded by an electron swarm. After elucidation of physical sense of the Semenov - Polanyi Rules, we comprehended that chemical properties of substances, their reactivity are determined by the difference between the energy of bond formed and broken during reaction. Qualitative coincidence of experimental data and model calculations showed that the chemical bond energy is determined by the first ionization potentials of atoms. Experimental data on the first ionization potentials periodically changed when nuclear charge increased. I.e., due to solution of a question "how chemical bond form and how chemical reaction proceeds", we managed to understand and explain the physical sense of the Periodic Law. |